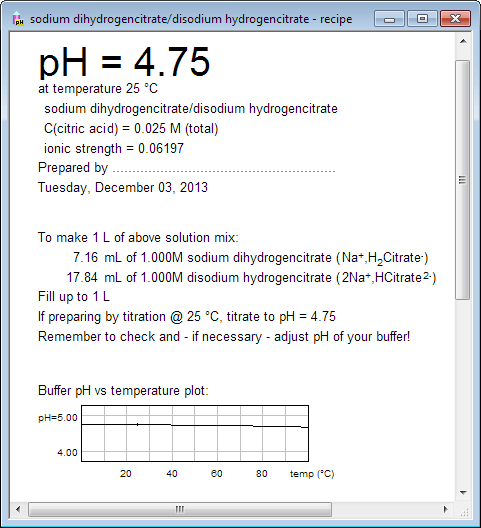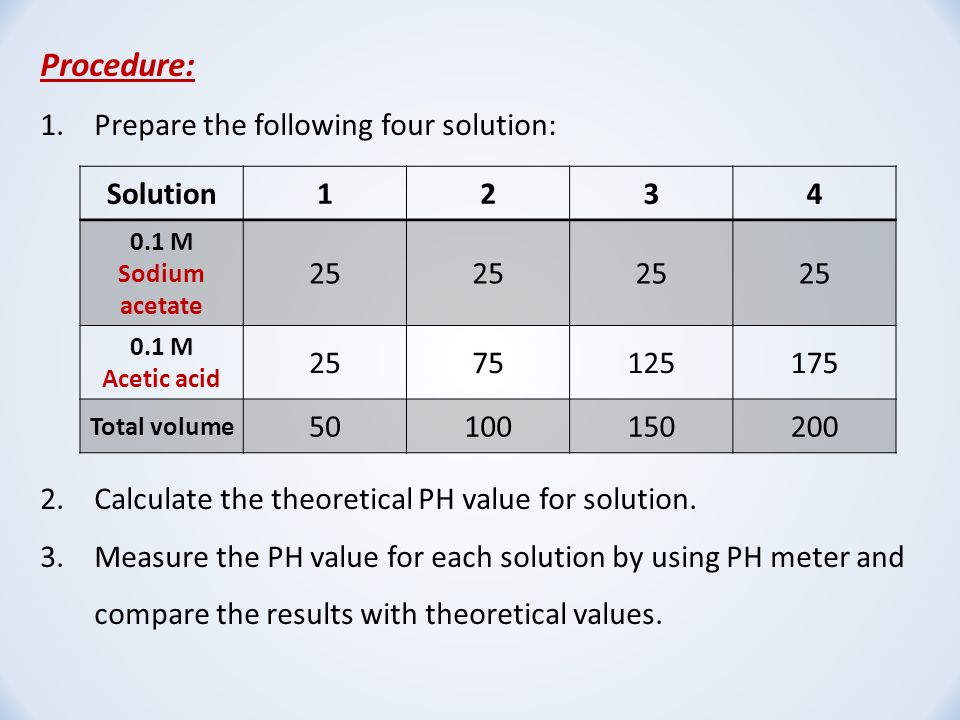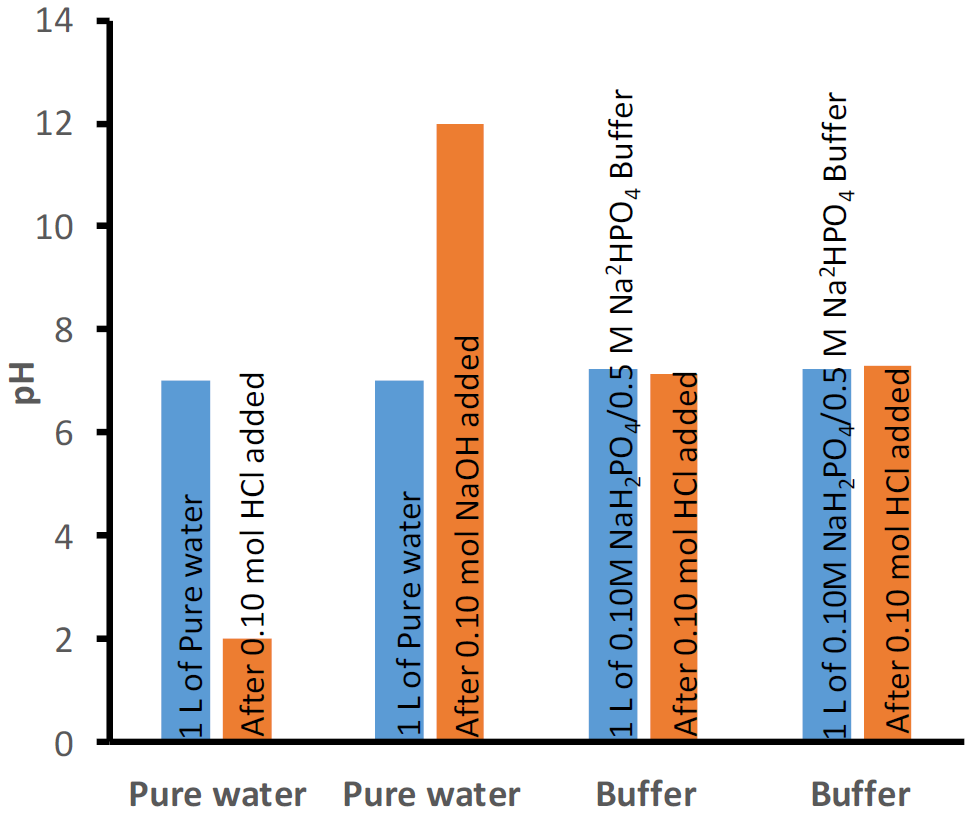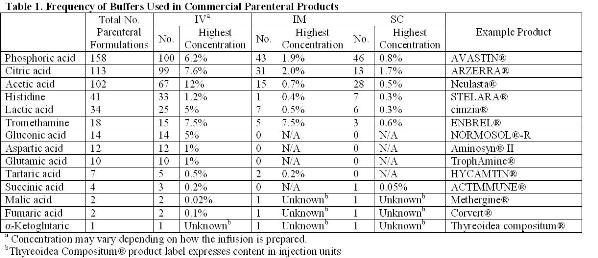
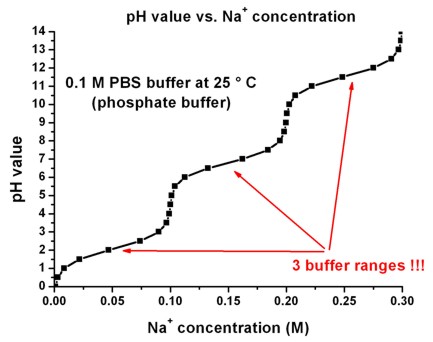
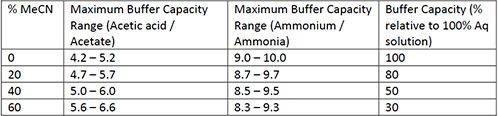
Breaking old habits: Moving away from commonly used buffers in pharmaceuticals - European Pharmaceutical Review

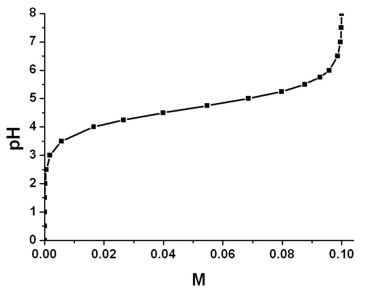
Effect of pH of 0.2 M acetate buffer on the absorbance value of 7μg/mL... | Download Scientific Diagram
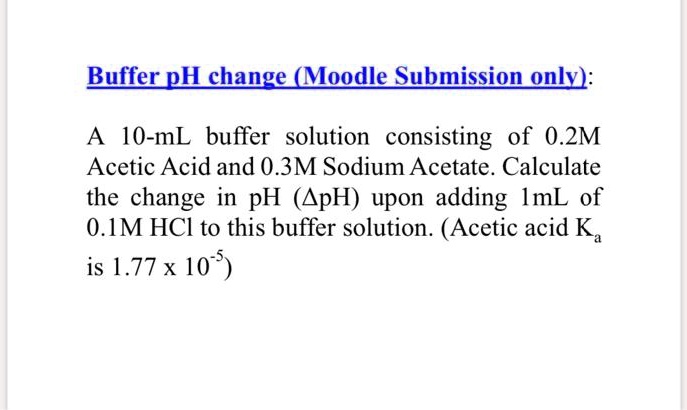
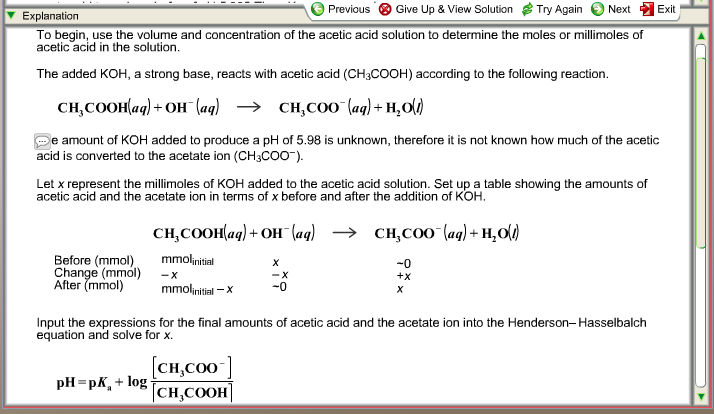
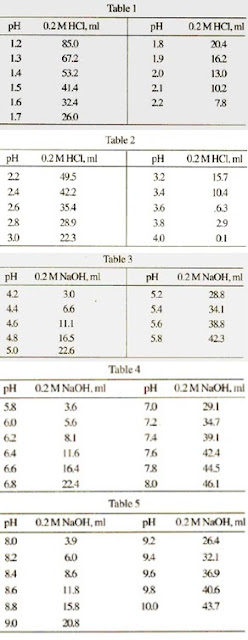
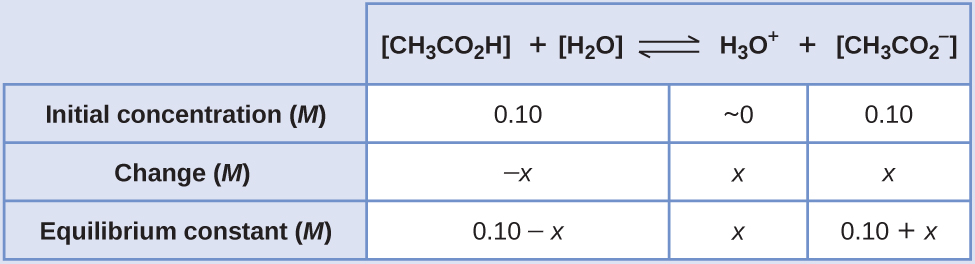
SOLVED: BufferpHchange (MogdleSubmission only): A 10-mL buffer solution consisting of 0.2M Acetic Acid and 0.3M Sodium Acetate. Calculate the change in pH (ApH) upon adding ImL of 0.1M HCI to this buffer




![Sodium Acetate [CH3COONa] Molecular Weight Calculation - Laboratory Notes Sodium Acetate [CH3COONa] Molecular Weight Calculation - Laboratory Notes](https://www.laboratorynotes.com/wp-content/uploads/2021/11/sodium-acetate-molecular-weight-calculation.jpg)

![BS093] 1.5M Sodium Acetate, pH 6.5 | Biosolution BS093] 1.5M Sodium Acetate, pH 6.5 | Biosolution](http://biosolution.cafe24.com/wp-content/uploads/2016/02/BS005-Sodium-Acetate-Solution.jpg)