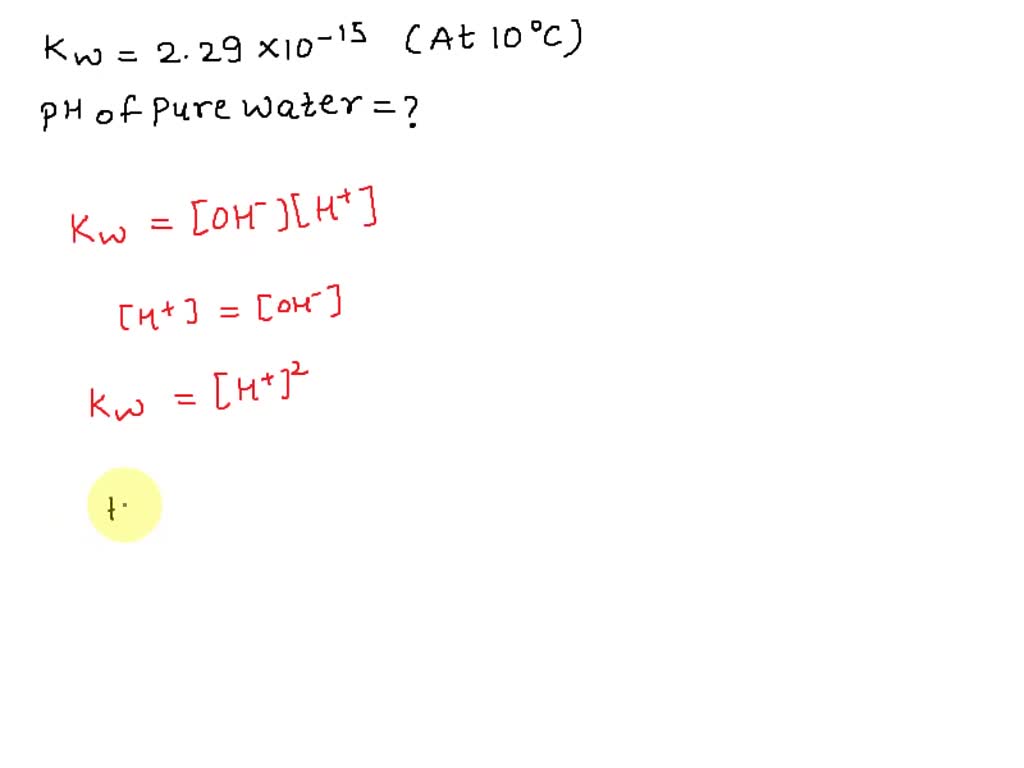The value of K_{W} is 9.55times 10^{-14} a certain temperature. Calculate the pH of water this temperature.

AutoIonization of Water, Ion Product Constant - Kw, Calculating H3O+, OH-, and pH Using Ice Tables - YouTube
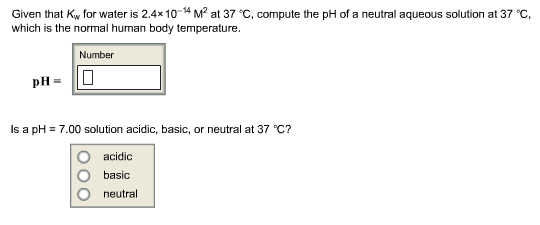
The ionization constant for water (Kw) is 9.311 × 10−14 at 60 °C. What is the [H3O+], [OH−], pH, and pOH for pure water at 60 °C? Thanks. - TopScience - Quora

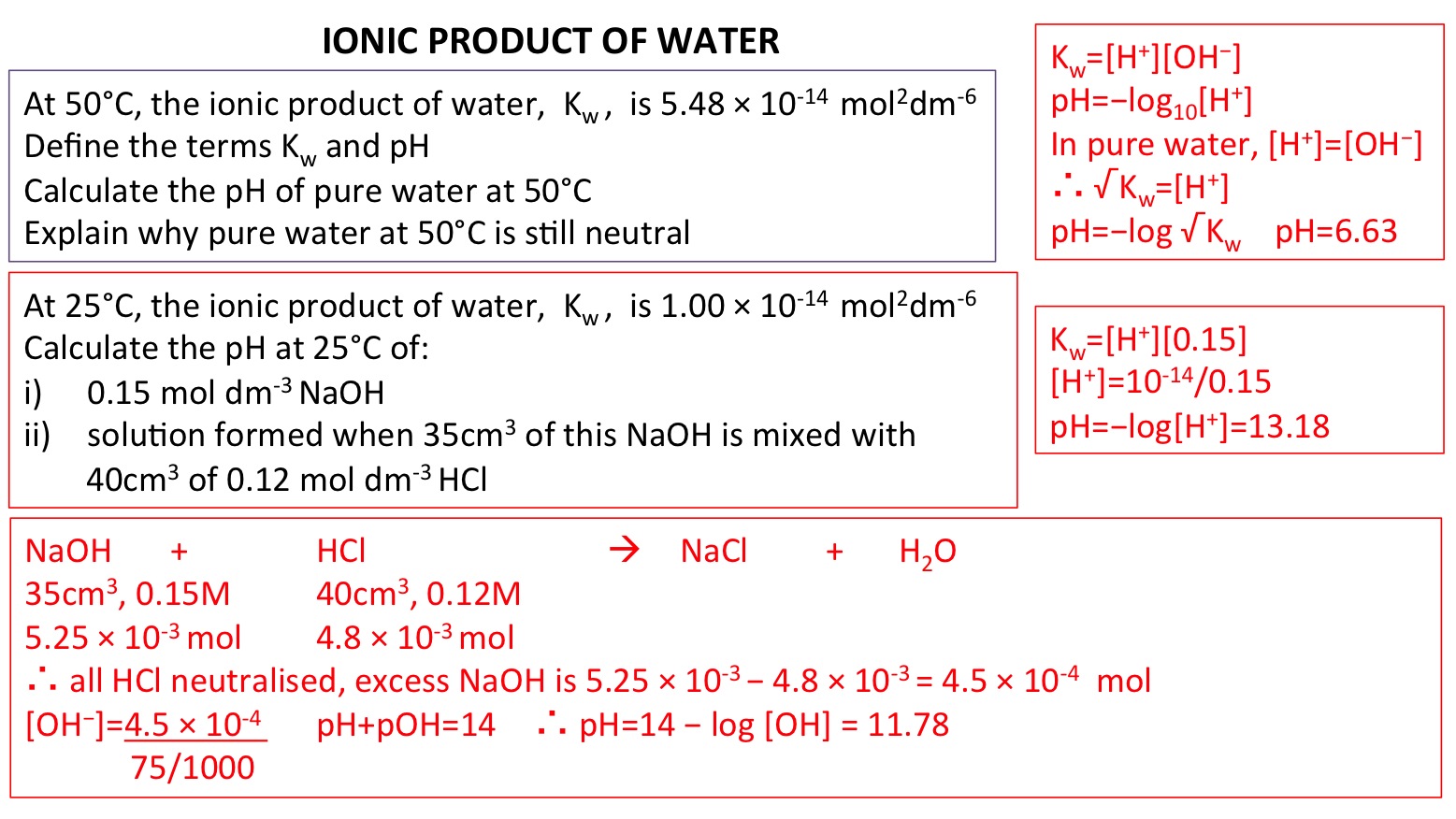
PPT - Kw and Temperature Energy + H 2 O ⇋ H + + OH - @ 25 o C Kw = 1.0 x 10 -14 Higher Temperatures @ 50 o C Kw PowerPoint Presentation - ID:1080833
![PPT - Definition pH and pOH. Given pH, pOH, [H 3 O + ] or [OH¯], calculate the remaining values. PowerPoint Presentation - ID:5054819 PPT - Definition pH and pOH. Given pH, pOH, [H 3 O + ] or [OH¯], calculate the remaining values. PowerPoint Presentation - ID:5054819](https://image2.slideserve.com/5054819/slide13-l.jpg)



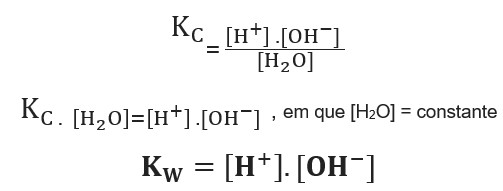
![Calculating [H₃O⁺] and pH (worked examples) (video) | Khan Academy Calculating [H₃O⁺] and pH (worked examples) (video) | Khan Academy](https://cdn.kastatic.org/ka_thumbnails_cache/e67920d2-30a0-40ef-bcfb-a7761c9673f6_1280_720_base.png)

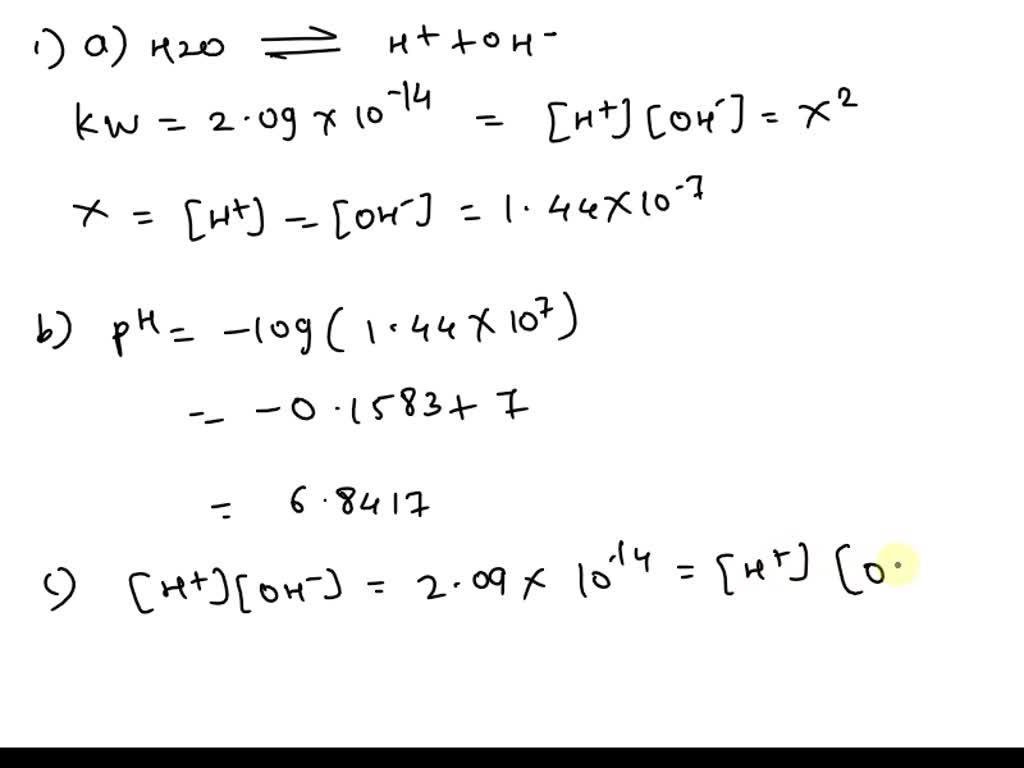
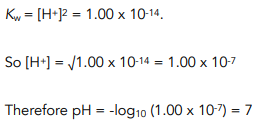




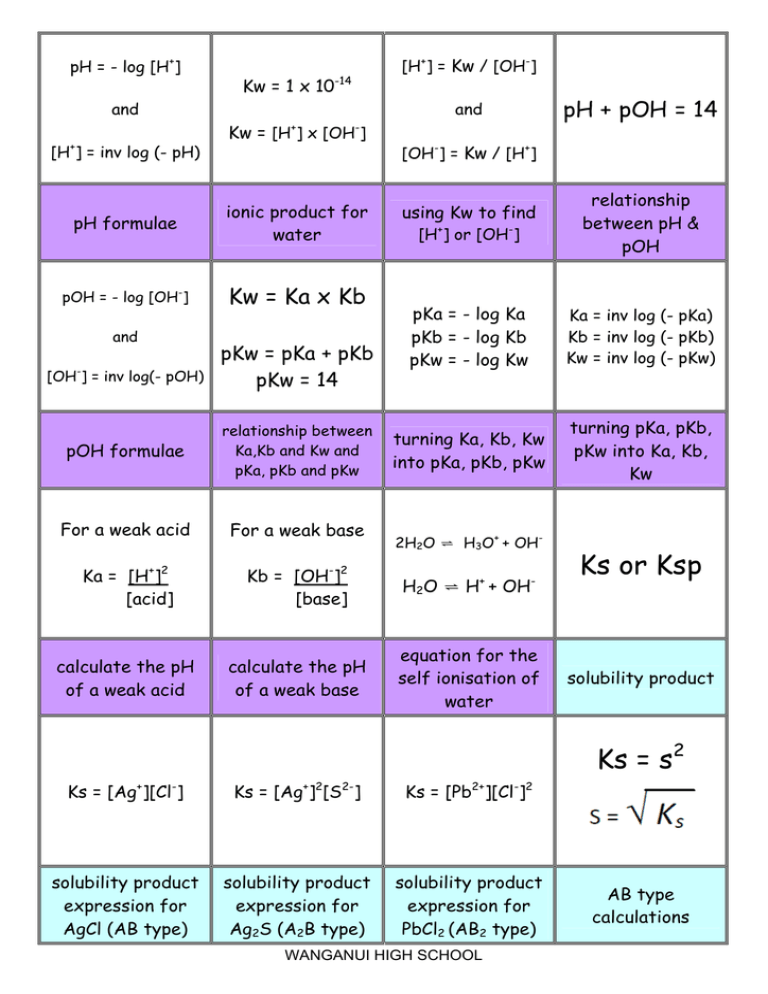
![Acids and Bases Part 4: Kw and Calculation of [H+] and [OH-] - YouTube Acids and Bases Part 4: Kw and Calculation of [H+] and [OH-] - YouTube](https://i.ytimg.com/vi/IvP_PxetNUw/maxresdefault.jpg)