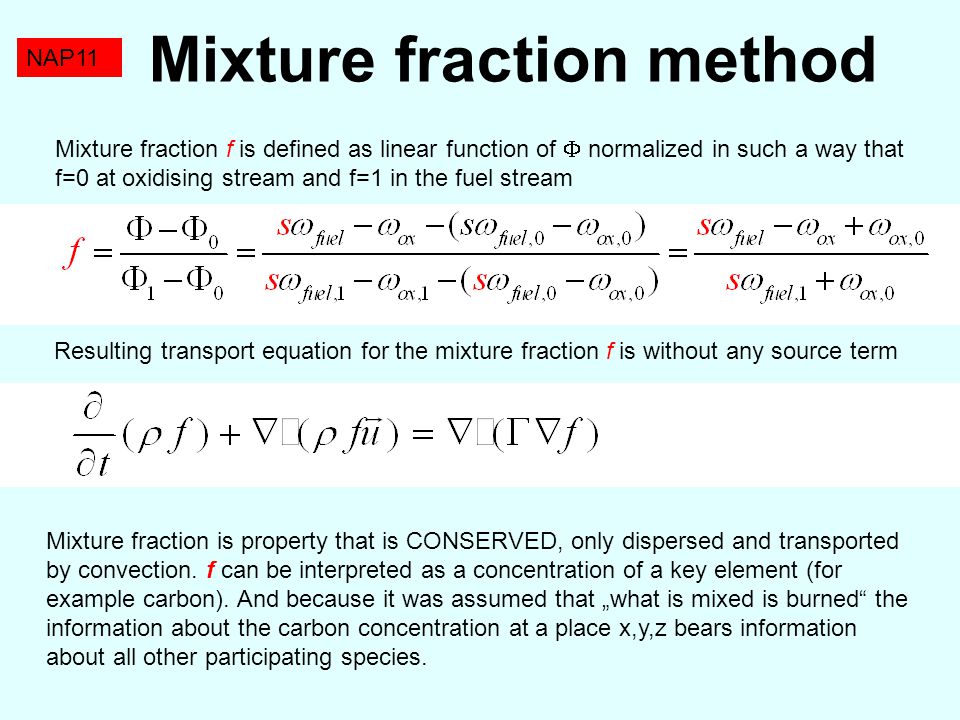
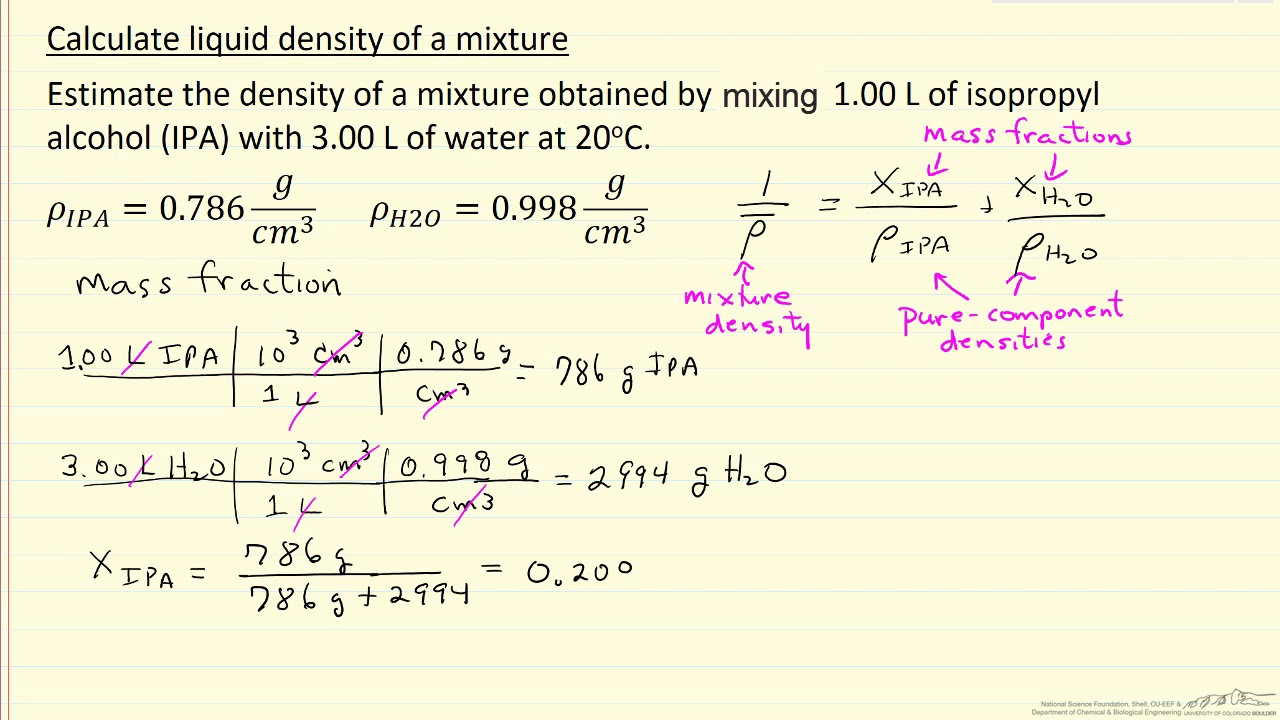A mixture has 18 g water and 414 g ethanol. The mole fraction of water in mixture is (assume ideal behaviour of the mixture) :
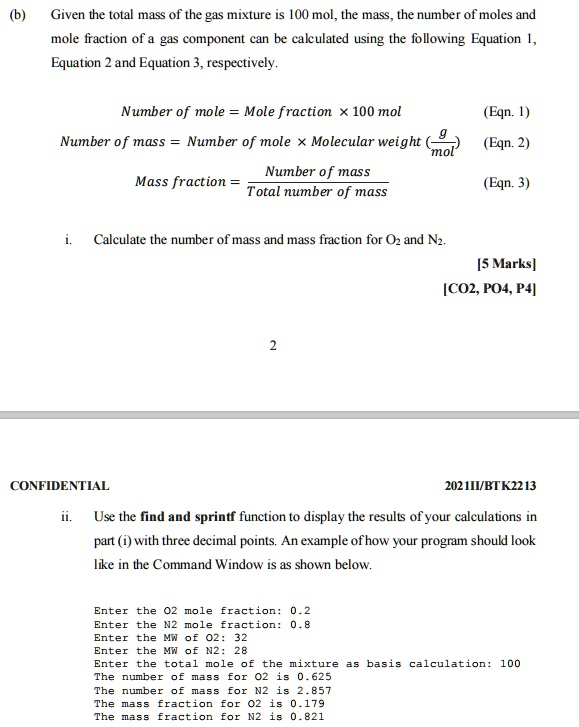
SOLVED: Given the total mass of the gas mixture 100 mol, the mass , the number of moles and mole faction of gas component can be cakulated using the following Equation 1,

LMI calculation: instantaneous value of the local heat release as a... | Download Scientific Diagram

Graph of mole fraction and temperature against mixture fraction from... | Download Scientific Diagram

CHEM 201 - Finding mole fraction from vapor pressure of a mixture with two volatile liquids - YouTube
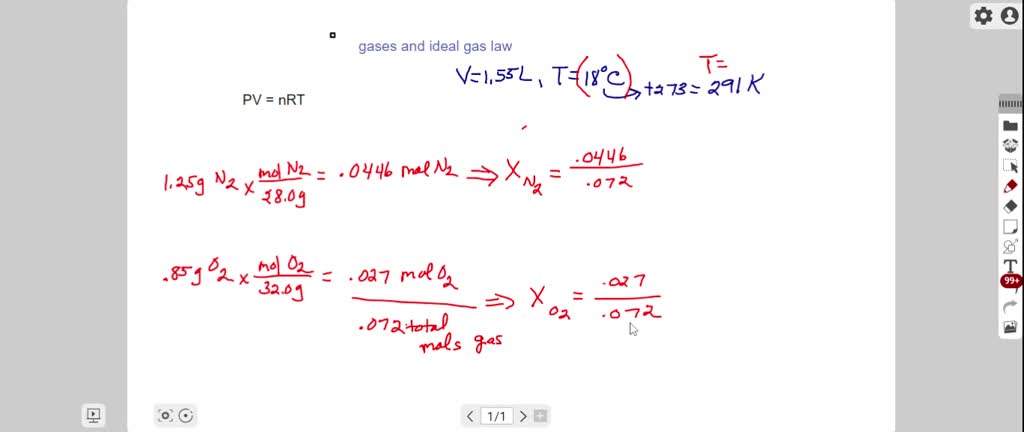
SOLVED: A gas mixture contains 1.25 g N2 and 0.85 g O2 in a 1.55-L container at 18 C. Calculate the mole fraction and partial pressure of each component in the gas mixture.